Download Risk Management Medical Devices Example created for productivity and performance. Perfect for students, specialists, and hectic households.
From basic daily plans to detailed weekly layouts, our templates help you remain on top of your top priorities with ease.
Risk Management Medical Devices Example
Risk Management Medical Devices Example
Beginner s Workout at a Glance Week 1 Full body split Week 2 Two day split Upper body Lower body Week 3 Three day split Push Pull Legs Week 4 Four day split Full body This 8 week workout program for beginners covers all of the basics needed to. Main Goal: Build Muscle. Time Per Workout: 30-45 Mins. build lean muscle mass! Link to Workout:.
START FROM SCRATCH THE COMPLETE BEGINNER

Use Of International Standard ISO 10993 1 Biological Evaluation Of
Risk Management Medical Devices Example · This 4-week workout for beginners combines walking and full-body strength training to tone your upper body, lower body and core and boost your metabolism. Week 7 8 Three weekly sessions 6 exercises per session with 3 4 sets per exercise Week 9 10 Four workout days per week 5 exercises each day with 3 sets per exercise Week 11 12 Training four days a week 5 6
This program involves training four times weekly two sessions for resistance training and one session each for Zone 2 and Zone 3 cardio Resistance training will help you Medical Device Risk Assessment Template Siamptu · Free Workouts & Advice. 12 Week Beginners Training Routine designed by Doug Lawrenson from Muscle & Strength. Use this workout to.
STARTING STRONG THE ULTIMATE 8 WEEK WORKOUT FOR
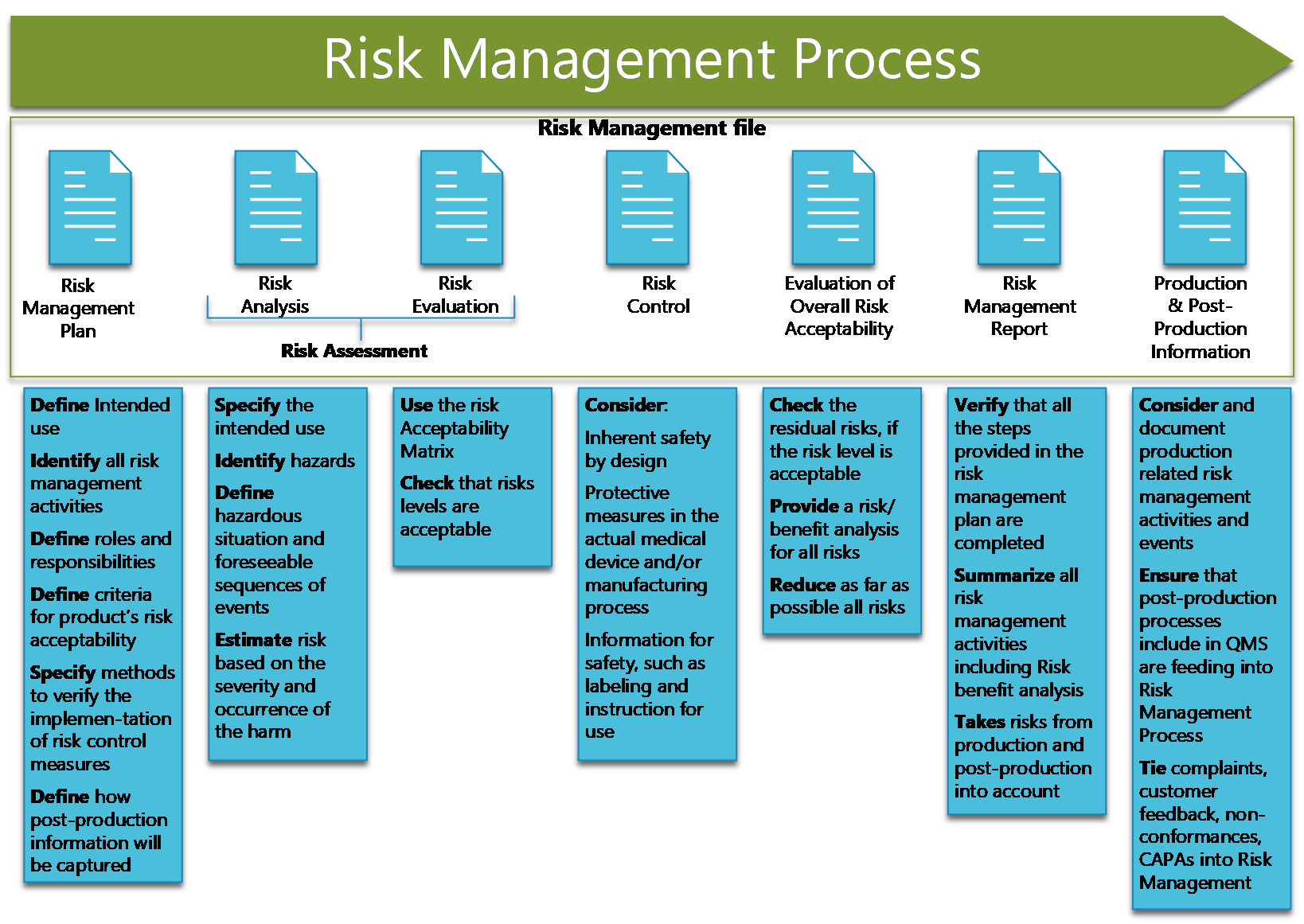
Risk Assessment Management
September 14 2022 If you re serious about building big muscles getting strong or simply taking your toning to the next level your quest will lead you to the gym Home bodyweight workouts Risk Management For Medical Devices ISO 14971 Compliance
Map to help you learn how to build muscle the right way Link to Workout https www muscleandstrength work outs start from scratch beginner workout Main Medical Device Risk Assessment Template Brasilmsa Dirk H ber Head Quality Senior Consultant Congenius AG XING

Articles Archives Medical Device HQ

Operational Excellence ProductLife Consulting

Risk Management MR comp GmbH

Marta Petrova Product Owner Msg Life Central Europe Gmbh XING
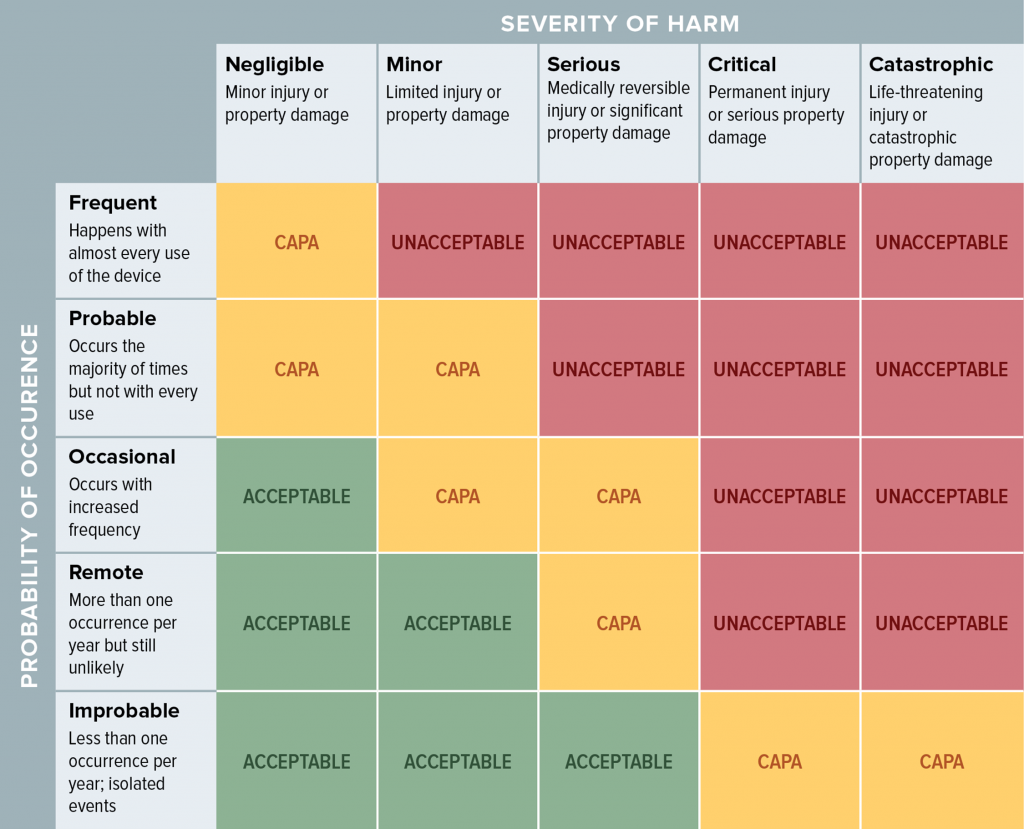
Residual Risk
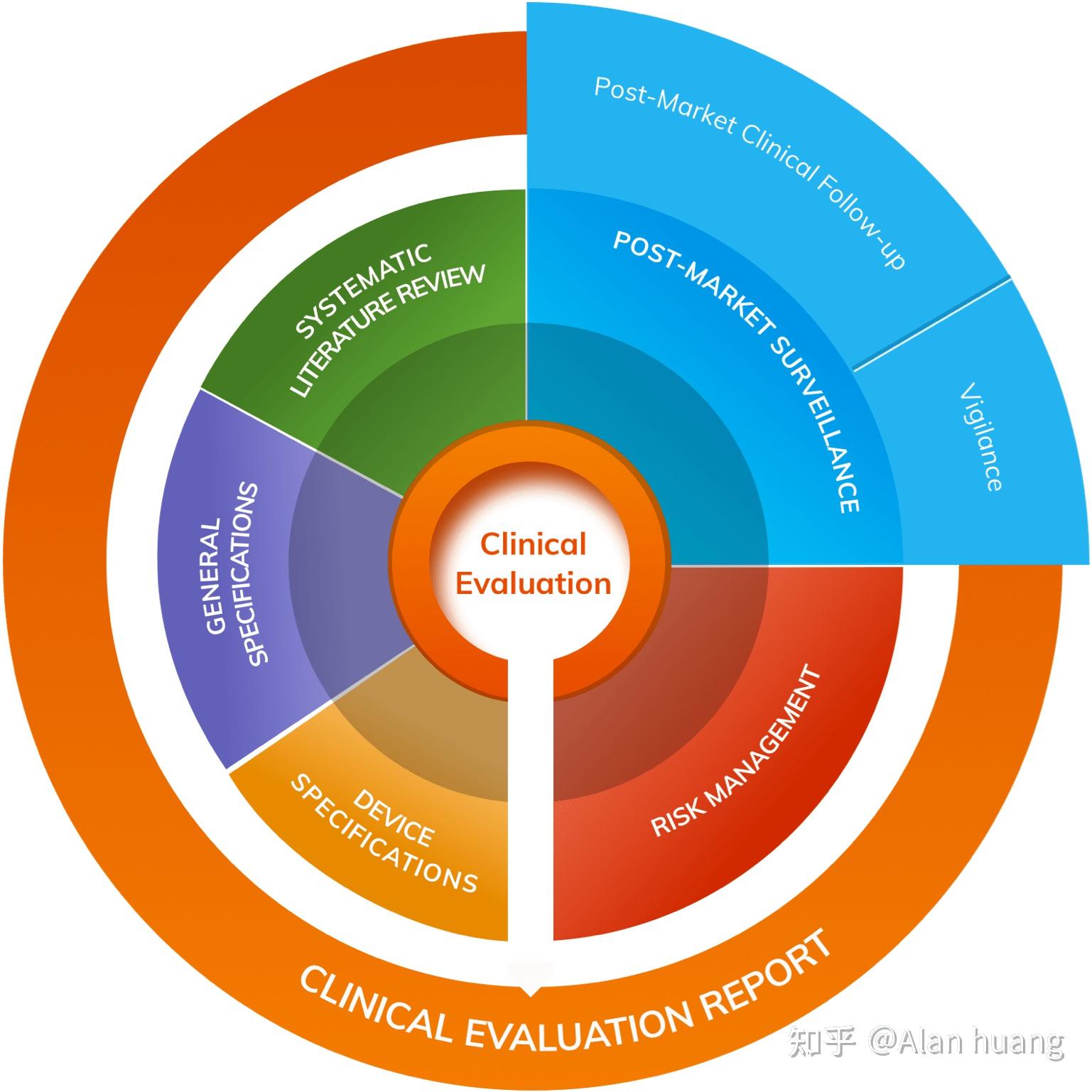
MDR CER

Risk Management Medical Devices Certification Experts

Risk Management For Medical Devices ISO 14971 Compliance
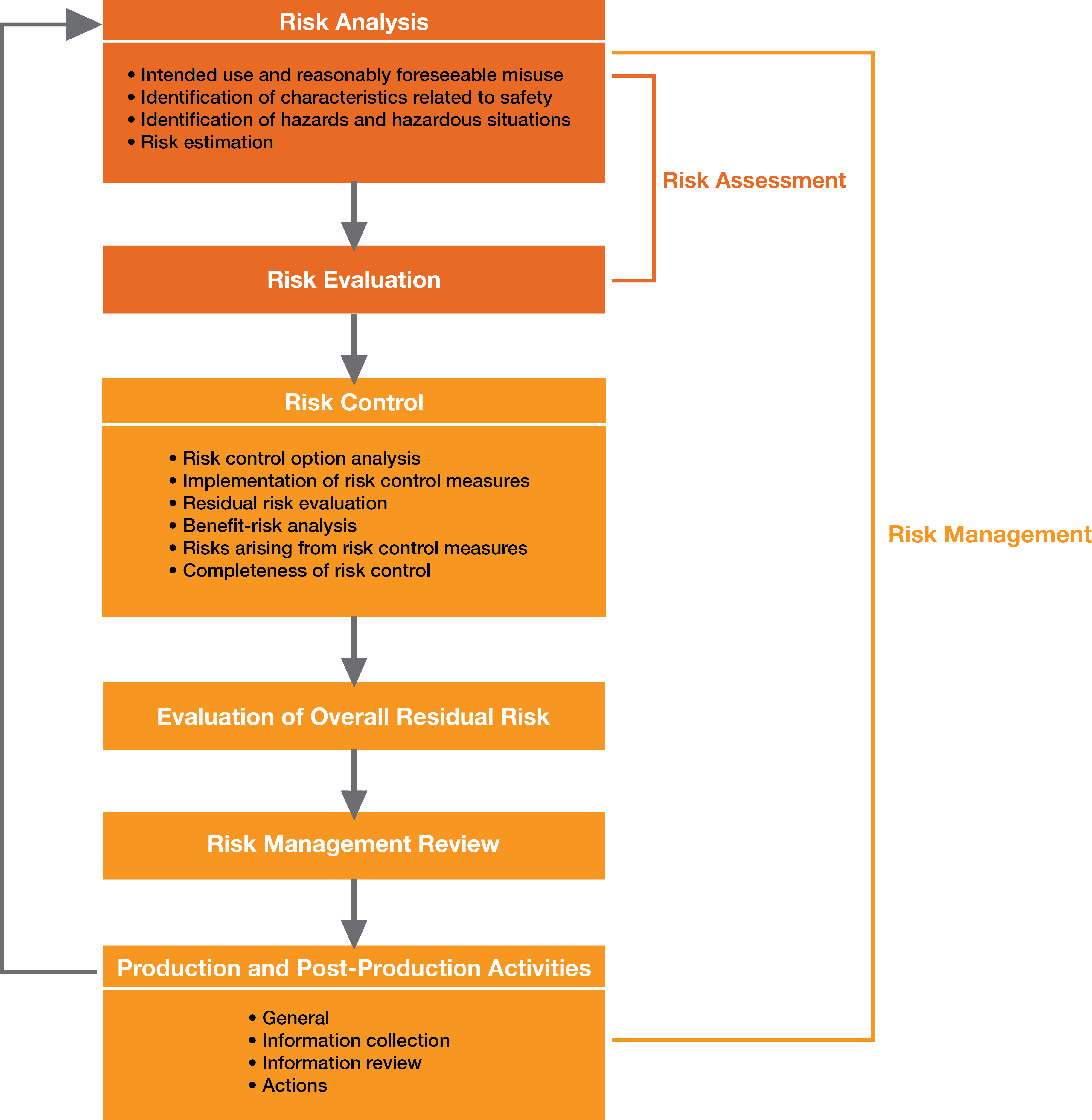
Medical Device Risk Assessment Template Dsaefab
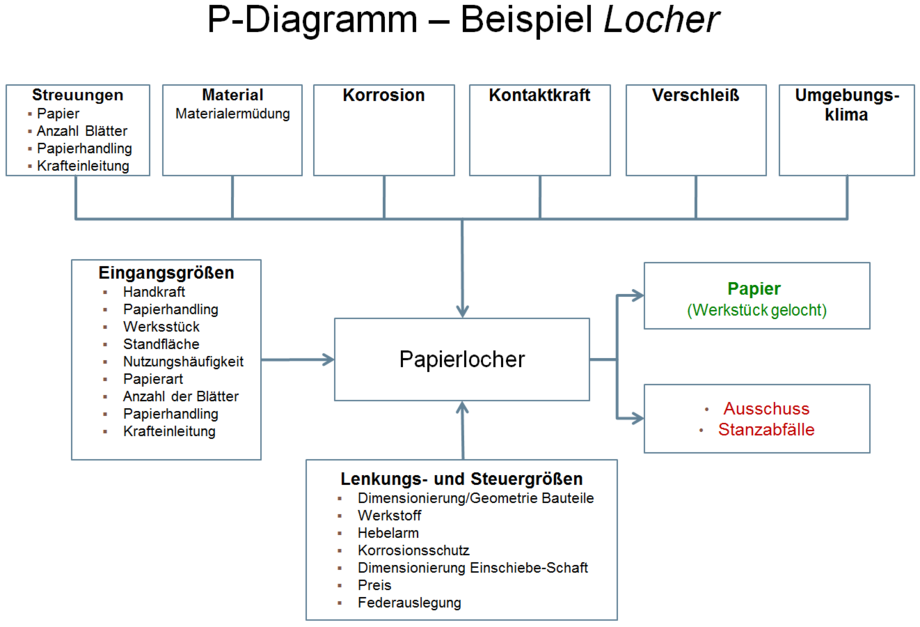
Fachbeitr ge FMEA Parameter Diagramm Dietz Consultants
