Download Application Of Risk Management To Medical Devices created for productivity and performance. Perfect for trainees, experts, and hectic households.
From easy daily plans to comprehensive weekly layouts, our templates assist you stay on top of your concerns with ease.
Application Of Risk Management To Medical Devices

Application Of Risk Management To Medical Devices
[desc_5]

Application Of Risk Management To Medical Devices In The EU PDF
Application Of Risk Management To Medical Devices[desc_6]
Technical Documentation Vee Care Asia [desc_3]

Application Of Risk Management To Medical Devices In The EU PDF
ISO 14971 2019 Risk Management Standard
ISO Medical Devices The Basics Percutaneous Heart Valves Standards And Modeling Timothy Kelley MS

ICTQual ISO 14971 2019 Medical Devices Application Of Risk Management

Medical Devices ISO 14971 Risk Management YouTube

What Is ISO 14971 2019 Application Of Risk Management To Medical

Resources Emet MedTech
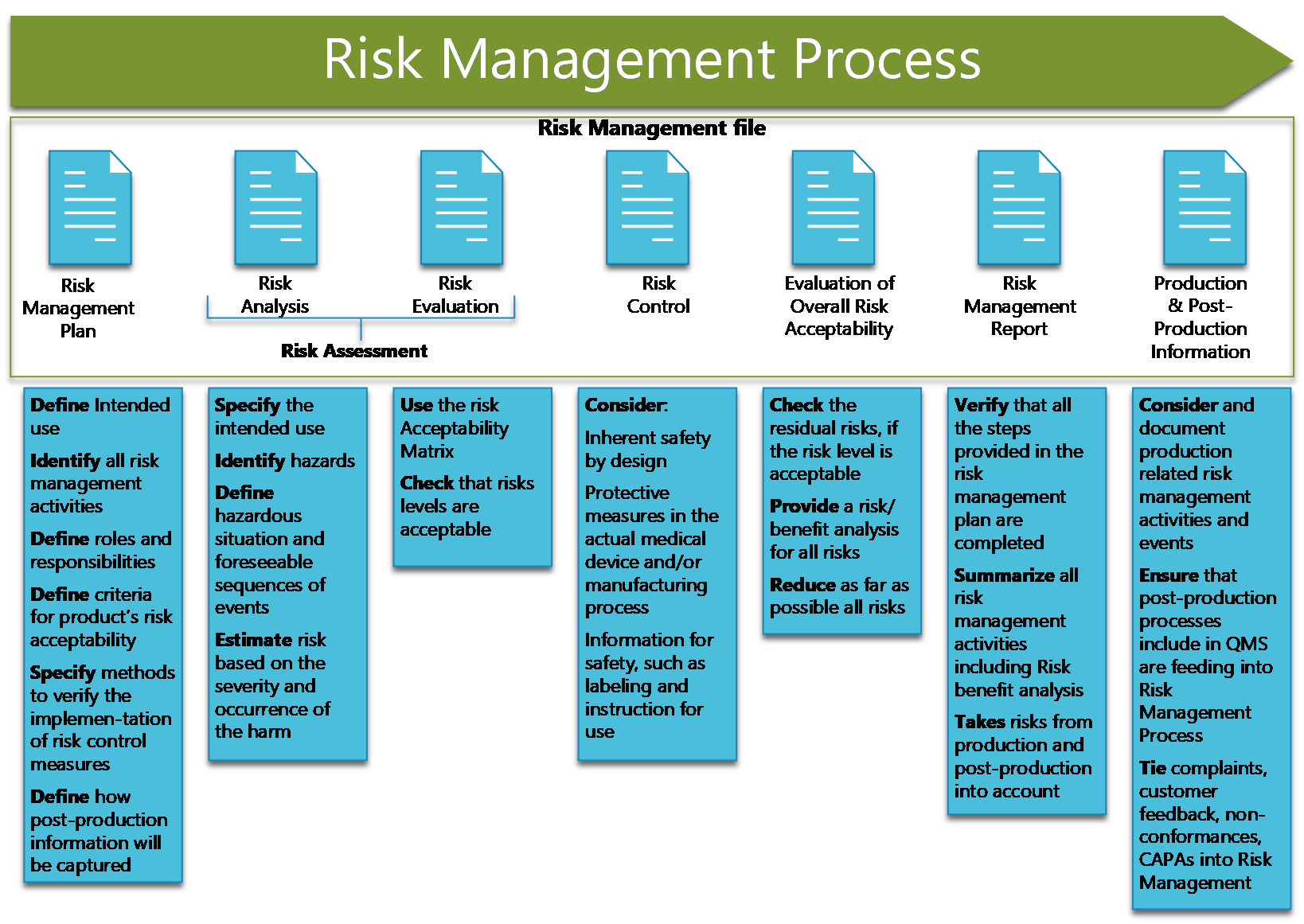
Risk Assessment Management
![]()
Resources SAMD Compliance

Webinar Risk Management In SaMD Development
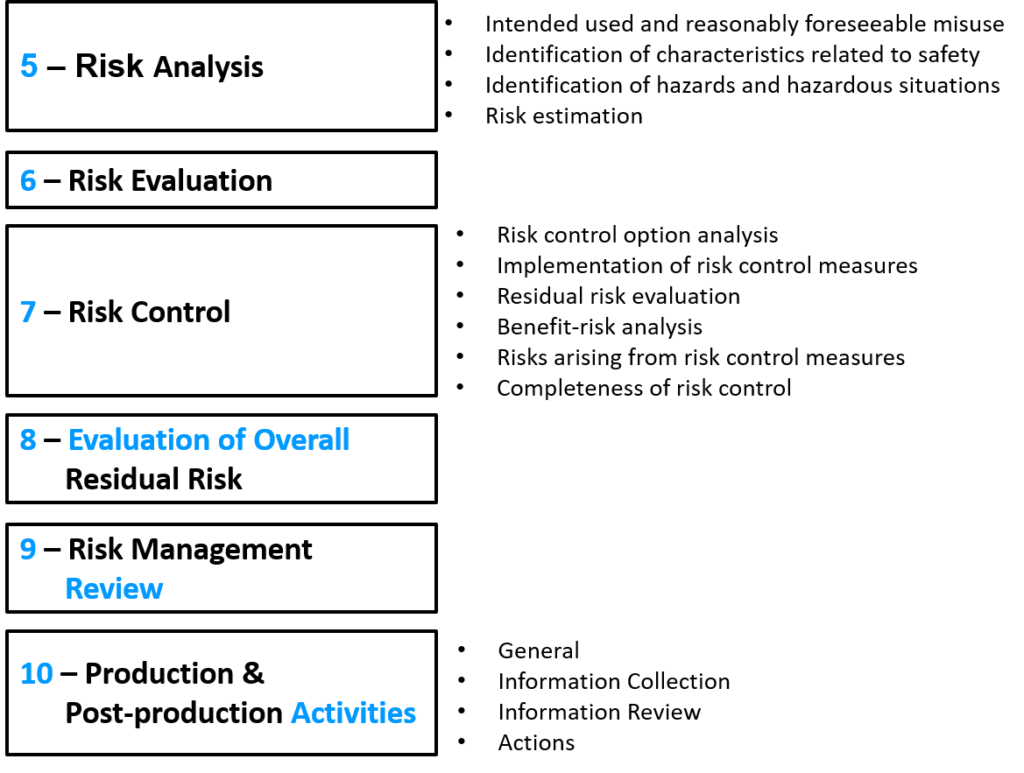
ISO 14971 2019 Risk Management Standard

EIFU Resources Regulations And Guidelines IFUcare

EIFU Resources Regulations And Guidelines IFUcare